Texas Planned Parenthood affiliates refuse to return COVID-19 relief funds

Parenthood affiliates in Texas say they have no intention of returning funds issued to them under the Small Business Administration’s (SBA) COVID-19 relief package, despite their ineligibility to receive the aid. The loans were distributed as part of the Paycheck Protection Program (PPP) under the CARES Act. As previously reported, 43 Planned Parenthood affiliates stole over $80 million in PPP funds knowing they had been disqualified from receiving the loans.
According to Department of the Treasury records, three Texas Planned Parenthood affiliates received loans. To date, only Planned Parenthood of Greater Texas has returned its loan, which was listed at between $2 million and $5 million.
The two remaining affiliates, Houston-based Planned Parenthood Gulf Coast ($2M to $5M) and Planned Parenthood South Texas ($350K to $1M), located in San Antonio, have refused to return the funds. “Together, the three Texas Planned Parenthoods brought in more than $62.7 million in revenue in 2018, according to their latest filings with the Internal Revenue Service,” reported the Dallas Morning News.
The two affiliates do not appear to be short on cash. Planned Parenthood South Texas amassed over $13 million in total assets by the end of 2018, and Planned Parenthood Gulf Coast spent $13 million on salaries alone in 2017, taking in $23 million in total revenue.
READ: Congressional Panel refers Planned Parenthood Gulf Coast for criminal prosecution
Planned Parenthood Gulf Coast made headlines in recent years when a representative was caught on undercover video by the Center for Medical Progress (CMP) discussing how the affiliate could make the sale of aborted baby body parts look legitimate (“just a matter of line items). In 2016, a Congressional Panel referred the affiliate to the Texas Attorney General, claiming PPGC “may have violated both Texas Law and U.S. Law when it sold fetal tissue to the University of Texas.” A procurement agreement from BioMax (below) reveals how profitable the harvesting of fetal organs can be for Planned Parenthood.

Service agreement to CMP from BIOMAX aborted fetal tissue from Planned Parenthood Gulf Coast
In March, Planned Parenthood Action, the corporation’s political arm, acknowledged that the CARES Act granted the Small Business Administration “broad discretion to exclude Planned Parenthood affiliates… under the new small business loan program. In addition, the bill attaches a new unnecessary Hyde Amendment provision….”
However, after multiple affiliates applied for and received the loans anyway, a group of Senators sent a letter to Attorney General William Barr calling for an investigation of Planned Parenthood, citing a rule which exempted the abortion corporation from receiving the federal dollars. “Trump administration officials were also quoted in public reports, explicitly clarifying that the SBA’s interim final rule ensured that no funds from the Paycheck Protection Program could go to Planned Parenthood,” the letter states.
The SBA has sent demand letters to the Planned Parenthood affiliates ordering them to give back the money. According to the New York Post, “They warned that ‘severe penalties’ were possible and that incorrect or false eligibility certifications for the stimulus funds could lead to criminal or civil sanctions if the SBA determines the borrowers knowingly made false statements.”
According to the Washington Post, the SBA pointed out that Planned Parenthood’s affiliates are not independent businesses:
In demanding money back from the Planned Parenthood affiliates, the SBA said the local organizations are little more than tendrils of a powerful national organization.
The Planned Parenthood Federation of America “is known to have and to exercise control over its local affiliates,” the SBA wrote in a letter to a local affiliate, which was subsequently posted online by NPR.
Citing a publicly available version of the national organization’s bylaws, the SBA noted that Planned Parenthood’s local organizations must submit to rigorous accreditation reviews every three years and can be stripped of their affiliation if they don’t maintain certain standards.
However, according to the Post, “Planned Parenthood rejected that description of its organization, noting that the bylaws themselves have to be approved by the local chapters. In a memo distributed to reporters, it noted that the national organization ‘has its own Board of Directors and management team, which is different from the Boards and management teams of the member organizations.’ ”
Stephanie Fraim, the president and CEO of Planned Parenthood of Southwest and Central Florida, admitted to NPR that her affiliate received $2 million in PPP loans to cover employee paychecks. Planned Parenthood of Metropolitan Washington (PPMW) received $1.3 million, according to a demand letter to PPMW from the SBA and Planned Parenthood of Delaware received close to $500K.
READ: Confirmed: 43 Planned Parenthood affiliates received COVID-19 relief funding
“The largest single government loan was a $7.5 million allotment to the Planned Parenthood of Orange and San Bernardino Counties in California.,” Fox News previously reported.
During the initial wave of COVID-19, many Planned Parenthood affiliates were allowed to remain open as “essential” to provide abortions while other businesses considered “non-essential” were forced to close.
Planned Parenthood’s latest 2018-19 annual report revealed that the taxpayer-funded abortion corporation accumulated $110 million in excess revenue for that year, making Planned Parenthood’s net assets for 2018 nearly $2 billion. The corporation committed a staggering 345,675 abortions. Recently accused of systemic racism, Planned Parenthood now garners 40 percent of the abortion market share. In the past decade alone (2010 to 2018) Planned Parenthood took in nearly $5 billion from U.S. taxpayers while committing nearly three million abortions.
“The Paycheck Protection Program… was designed by Congress to help struggling small businesses and nonprofit organizations by giving them access to low-cost loans… during this pandemic. It was not designed to give government funds to politicized, partisan abortion providers like Planned Parenthood,” lawmakers stated in their letter to the U.S. Attorney General. “Planned Parenthood fraudulently taking tens of millions of dollars that were intended to help keep those small businesses and nonprofit organizations afloat cannot stand and must be addressed.”
Reprinted with permission from Live Action News / 8/13/2020 Updated.
ADDITIONAL:
Records on file at the Department of the Treasury as of the end of June 2020 show the following Planned Parenthood’s received over $150K from the PPP program:
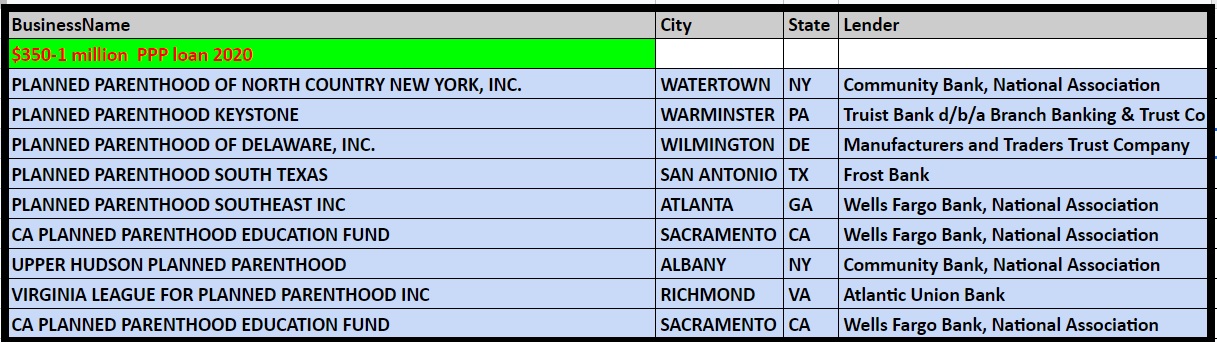
Planned Parenthood PPP loan from SBA thru June 2020 from US Treasury $350 to 1 million
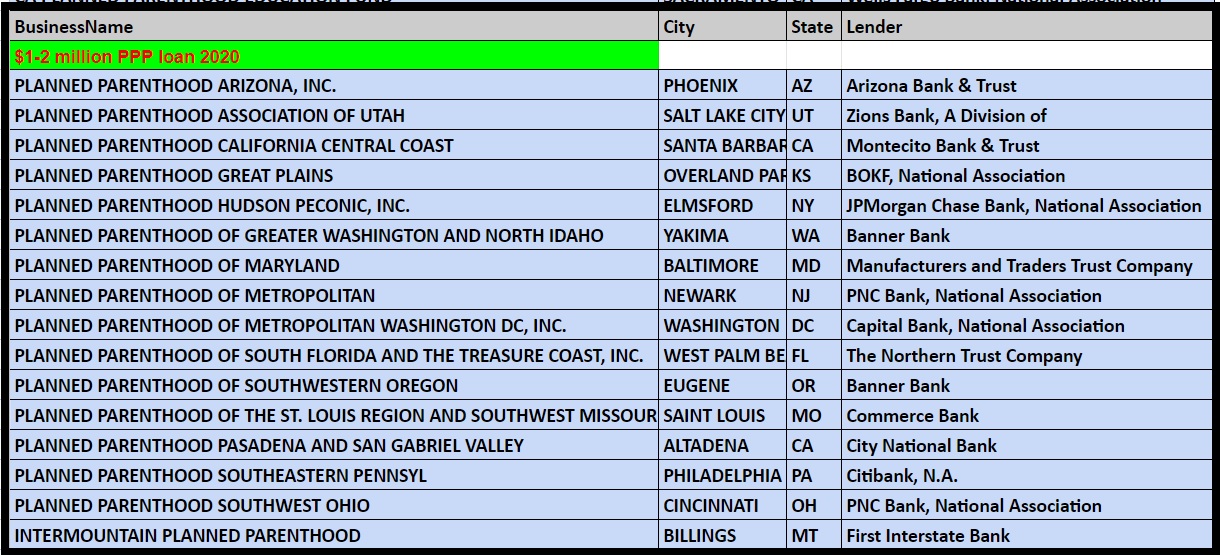
Planned Parenthood PPP loan from SBA thru June 2020 from US Treasury $1 to $2 million dollars
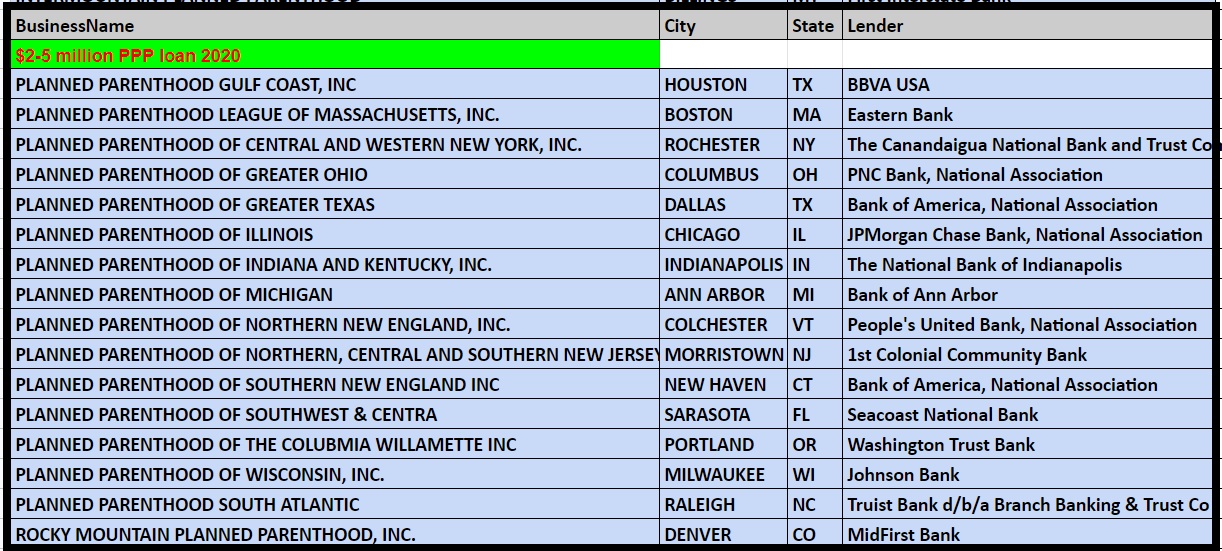
Planned Parenthood PPP loan from SBA thru June 2020 from US Treasury $2 to $5 million dollars
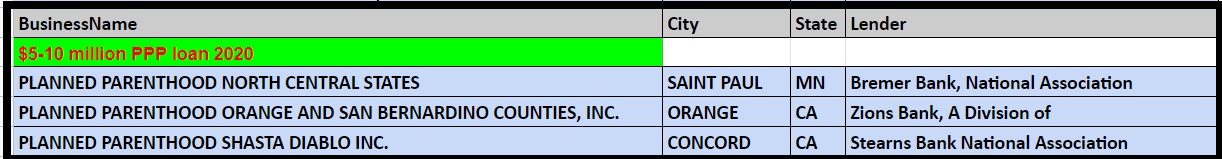
Planned Parenthood PPP loan from SBA thru June 2020 from US Treasury $5 to $10 million dollars
In March 2020, despite a shortage of personal protection equipment (PPE), Planned Parenthood began asking for PPE donations. In April 2020, an appeals court ruled that Planned Parenthood in Texas could commit abortions despite the pandemic.
In 2019, Planned Parenthood South Texas committed 1,855 abortions, 111 (6%) of which were performed after the first trimester. By the end of 2018, the affiliate had amassed over $13 million in total assets. Planned Parenthood Gulf Coast’s (PPGC) 2017 990 tax document reveals they took in $23 million in total revenue, spending $13 million on salaries alone. This included over $300K to PPGC’s CEO, Melaney Linton. In all, PPGC reported over $46 million in net assets or fund balances.